Title: Contractive Annulation – A New Strategy for the Synthesis of Small, Strained Cyclophanes
Speaker: Prof. Graham J. Bodwell, Chemistry Department, Memorial University of Newfoundland, St. John's, Canada
Time: Nov 22nd, Friday, 12:30-1:30pm
Venue: Building 24#-C205
Host: Prof. Jay Siegel
Abstract:
Small, strained cyclophanes are especially interesting because of the deviation of various structural features from ideality and the unusual spectroscopic and physical properties that emerge. Of course, the strained nature of such systems renders them challenging synthetic targets and the level of challenge tends to increase with the level of strain in the cyclophane. There is currently only one general synthetic strategy for accessing the most highly strained cyclophanes, i.e. the conversion of a bridged pre-arene with relatively low strain to the corresponding bridged arene (cyclophane) with high strain. The success of this approach is rooted in the gain of aromatic stabilization energy (ASE) that accompanies the conversion of the pre-arene to the arene, which serves to counterbalance the accumulating strain.
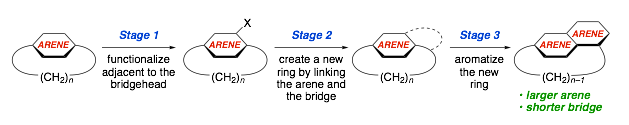
A new strategy for the synthesis of small, highly strained cyclophanes has been developed. It is a three-stage process, which commences with an existing small cyclophane. The first step is to functionalize the aromatic system at a position adjacent to one of the bridgeheads. This is followed by synthetic manipulation that leads to the formation of a new six-membered ring linking the arene to the benzylic position. Finally, the new ring is aromatized to afford a new cyclophane, which has both a larger aromatic system and a shorter bridge than the starting cyclophane. In essence, the bridge is being used as food for the growth of the aromatic system. The gain of ASE again plays a role in offsetting the increase in strain in the final step.