The Nobel Prize in Chemistry was jointly awarded to Emmanuelle Charpentier and Jennifer Doudna for their CRISPR work. It is the first time a science Nobel has been awarded to two women. The function of CRISPR was first identified in 2007 by researchers at the Danish yogurt company DANISCO. Lactobacillus responsible for yogurt fermentation are susceptible to viral infection, but they discovered that certain lactic acid bacteria behave as if they were resistant to viruses. Genomic analysis these bacteria revealed that CRISPR genes defeat the viral infection.
How CRISPR-Cas protects the immune system was not exactly known until Jennifer Doudna and Emmanuelle Charpentier elucidated CRISPR's mechanism (Science 2012, 337:816–821. doi: 10.1126/science.1225829). In brief, bacteria cut the invading virus's nucleotide sequence and store it in the CRISPR genes. When the virus bacteriophage re-invades, the viral nucleotide sequence stored in the CRISPR genes is transcribed to RNA and binds to the Cas9 protein to cleave invading viral DNA.
During viral infections, the effector complex recognizes short motifs near the target sequence in the virus and binds with sequences complementary to crRNA, initiating the Cas protein to break the viral genome by causing a double-stranded break (See figure). The double-stranded break caused by the Cas can be repaired with its repair mechanisms, such as non-homologous end joining (deletion or insertion) or homologous recombination (error-free recombination) pathway.
These findings enabled CRISPR as a new genetic scissors that could replace the existing lower efficiency and specificity genetic-scissor technologies such as ZFNs and TALENs. With CRISPR it is easy to engineer specific sequence binding, and simple to effect gene deletions, insertions and modifications. Numerous studies using various experimental models have contributed significantly to the high efficiency of CRISPR genome editing (Current Protocols in Molecular Biology. 2019, Sept 24. https://doi.org/10.1002/cpmb.106). Moreover, the simplicity and robustness of CRISPR have extended its application from basic sciences to translational research and medicine within a relatively short time (Biomedicines. 2018, Nov 12;6(4):105. doi: 10.3390/biomedicines6040105).
Gene editing using the CRISPR/Cas9 offers the potential to alter humankind in ways yet not feasible; it is imperative to ensure international standards for its ethical use. With proper cautions, CRISPR technology holds the promise to correct genetic diseases and enhance current therapies beyond imagination, the perfect harmony with Alfred Nobel's bequest.
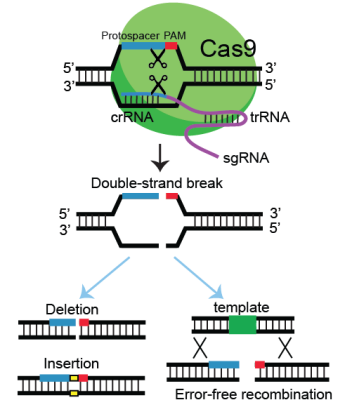
Figure. Schematic representation of the CRISPR-Cas9 genome editing.
Double-strand breaks generated by Cas9 guided with RNA molecules. The breaks are repaired via error-prone (insertion or deletion) or error-free recombination. Yellow box represents small nucleotide insertion and green box represents insertion of GFP tag. This figure is modified from Current Protocols in Molecular Biology by Kim and Colaiacovo (2019).
Written by Hyun M. Kim, SPST, TJU

