Nowadays, associate professor Nan Li and her team from the School of Pharmaceutical Science and Technology published an article on novel nanomaterials for combined photothermal therapy (PTT) and ferrotherapy (FPT) in tumor treatment in Acta Pharmaceutica Sinica B, a leading journal of Chinese Science and Technology Journals. The title of the article is ‘Iron-based and BRD4-downregulated strategy for amplified ferroptosis based on pH-sensitive/NIR-II-boosted nano-matchbox’ (DOI: 10.1016/j.apsb.2022.05.011). In this paper, the matchbox-shaped nanomaterials were cleverly designed and endowed with tumor therapeutic effects (FIG.1).
The ferroptosis is a newly identified form of cell death, which is defined as the missed control of membrane lipid peroxidation (LPO) due to the inactivation of the glutathione peroxidase 4 (GPX4). Besides, the high levels of iron and ROS are the characteristics of FPT. However, the effect of FPT is largely limited by insufficient endogenous iron content and the presence of intracellular mechanisms for ROS clearance.
In this paper, the gold nanorods (GNRs) with NIR-II absorption capability were fabricated initially. Then, the pH-responsive metal-organic frameworks (MOFs) were coated onto the GNRs. Consequently, the nano-matchbox morphology of the nanoccarriers was cleverly observed (FIG. 2). Typically, this nano-matchbox was degradable in acidic tumor microenvironment (TME), which induced the releasing of loaded iron-supplements ammonium ferric citrate (FAC) and FPT-related protein inhibitor JQ1. On one hand, the FAC-induced Fenton/Fenton-like reactions in the TME can simultaneously generate iron (Fe3+/Fe2+) and ROS to initiate the FPT by LPO elevation. On the other hand, JQ1 as a small molecule inhibitor of BRD4 protein can amplify FPT through downregulating the expression of glutathione peroxidase 4 (GPX4).
At the same time, the internal match-shaped GNRs in nano-matchbox could realize the PTT effect through the local surface plasmon resonance (LSPR) and high photothermal conversion efficiency under the NIR-II laser irradiation. As a result, our study points out a PTT combined iron-based/BRD4-downregulated strategy for amplified ferrotherapy both in vitro and in vivo.
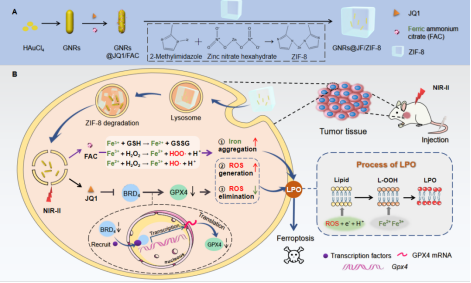
FIG.1 Schematic illustrations of (A) the synthesis route of GNRs@JF/ZIF-8 and (B) amplified FPT therapy.

FIG.2 TEM images of A) GNRs, B) ZIF-8, and C) GNRs@JF/ZIF-8.
Lujing Geng, the master student of School of Pharmaceutical Science and Technology, is the first author of this paper. School of Pharmaceutical Science and Technology of Tianjin University is the first corresponding address of this paper. Jiao Li, the associate professor of School of Precision Instruments and Optoelectronics Engineering of Tianjin University is the co-corresponding author of this paper. This work was supported by the National Natural Science Foundation of China, Foundation Young Elite Scientists Sponsorship Program by Tianjin, Major Special Projects of Tianjin, and General Program of Tianjin. This work was also supported by the analysis center of the School of Pharmaceutical Science and Technology.

