The clinical application of adriamycin has been hindered by the notorious irreversible cardiomyopathy that is dependent on the cumulative dose. It is reported that the maximum tolerated dose of adriamycin is 550 mg/m2, significantly affecting patient compliance and therapeutic outcomes. In 2019, the mechanisms of adriamycin-induced cardiotoxicity were revealed due to ferroptosis, which opens new avenues for alleviating cardiomyopathy via targeting ferroptosis. Ferroptosis is an iron-dependent cell death pathway driven by extensive lipid peroxidation in cell membranes. Since ferroptosis is highly dependent on iron, it was postulated that amphiphilic polymer iron chelators could efficiently address adriamycin-induced cardiac damage via ferroptosis suppression. Meanwhile, it was further presumed that helical iron-chelating polymers were more potent in ferroptosis inhibition and cardiotoxicity alleviation due to the prolonged retention in plasma membranes.
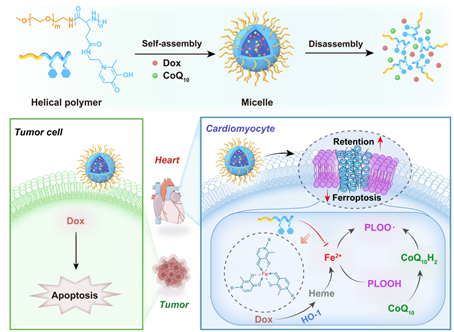
Figure 1. Helical polymer micelles for the alleviation of adriamycin cardiotoxicity
A project led by Prof. Yanjun Zhao and Prof. Zheng Wang at SPST has designed an intelligent appoach to address the above problem (Figure 1). They designed a helical iron-chelating polymer micelle that physically loaded adriamycin with a radical trapping agent, coenzyme Q10. Such design enables similar pharmacokinetics and biodistribution of all active agents and thus maximizes the effect of ferroptosis suppression on alleviating cardiomyopathy. The iron chelation in synergy with radical trapping could efficiently rescue cardiocytes post adriamycin challenge at both cellular and animal models. Meanwhile, ferroptosis inhibition did not affect the cytotoxicity of adriamycin against model 4T1 cancer cells. The delivery of adriamycin via iron-chelating polymer micelles did not affect the anti-tumor efficacy. Intriguingly, the alleviation of adriamycin-induced cardiotoxicity markedly increased the survival rate of 4T1 tumor-bearing mice, indicating that the management of adverse effect of adriamycin on cardiocytes might improve the therapeutic outcome. The current work provides a translational nanomedicine for the efficient management of adriamycin cardiotoxicity, which may find application in the efficacy enhancement of other types of anthracyclines without the hassle of cardiac adverse effects

