Cancer resistance is often associated with the presence of persister cells and the polarization of cancer cells towards a mesenchymal state. Therefore, ferroptotic cancer therapy has gained extensive attention because it is potent to eradicate resistant cancers that are refractory to conventional apoptosis-based approaches. Tailored phospholipids (arachidonoyl phosphatidylethanolamine/PE-AA) are the execution molecules of ferroptosis and their synthesis relies on the long-chain acyl-CoA synthetase 4 (ACSL4). However, the expession of ACSL4 in certain tumors is very low, resulting in ferroptosis resistance. To address this issue, the Zhao and Wang Group at SPST employed a ferrocene-bearing polymer micelles to co-deliver PE-AA and auranofin (Aur) to ACSL4-deficient breast cancer (MCF-7) in attempt to circumvent the ferroptosis resistance (Figure 1). Aur targets thioredoxin reductase and regulates redox homeostasis. The ferrocene in the micelles enabled the H2O2-responsive micelle disassembly and cargo release in the MCF-7 cells. Meanwhile, the Fenton-like reaction between ferrocene and H2O2 released the iron and produced reactive oxygen species; both could sensitize ferroptosis. The co-delivery of PE-AA and Aur potently induced ferroptosis in vitro and in vivo. In particular, PE-AA could circumvent the difficulty of ferroptotic lipid biosynthesis in ACSL4-deficient MCF-7 tumors. This work provides a facile approach to sensitize ferroptosis-resistant tumors to lipid peroxidation, which may expand the application of ferroptotic cancer therapy.
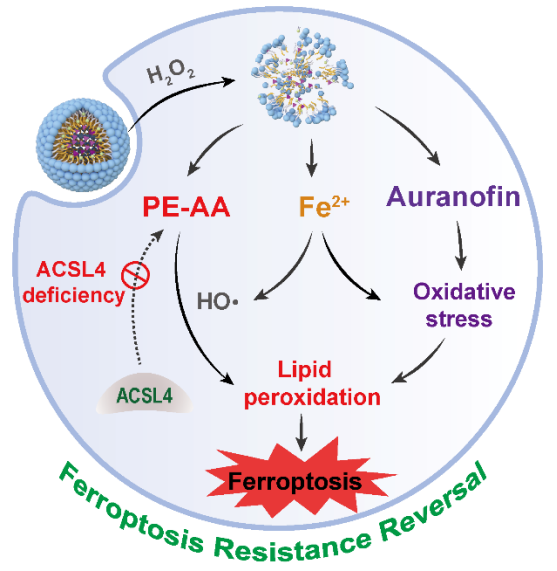
Fig 1. Reversing the ACSL4 deficiency-induced ferroptosis resistance via ferrocene-bearing polymer micelles.

