On November 18, 2025, Prof. Zhiguang YUCHI's research group at the State Key Laboratory of Synthetic Biology and the School of Pharmaceutical Science & Technology, Tianjin University, published a research article in Nature Communications titled “Crystal structures of Ryanodine Receptor reveal dantrolene and azumolene interactions guiding inhibitor development.” This study is the first to elucidate, at the molecular level, the fine regulatory mechanism of dantrolene, the only FDA-approved inhibitor of the ryanodine receptor (RyR). Based on these insights, the team also developed novel RyR inhibitors, providing a crucial mechanistic foundation for therapeutic development and green insecticide design targeting RyR.
RyRs are Ca²⁺ release channels on the endoplasmic reticulum membrane, essential for muscle excitation, cardiac rhythm regulation, and neuronal signaling. They are major drug targets for various inherited and acquired diseases and also serve as important targets for modern insecticides. Malignant hyperthermia (MH), caused by gain-of-function mutations in skeletal-muscle RyR1, leads to rapid onset of hyperthermia, muscle rigidity, and metabolic crisis when patients encounter anesthetics or heat stress, with extremely high mortality. In China, MH mortality reaches 73.5%. Dantrolene is the only approved RyR inhibitor and the standard treatment for MH, also used prophylactically before anesthesia. Additional clinical applications—such as relief of spasticity in cerebral palsy, spinal cord injury, multiple sclerosis, and stroke—have led to a domestic annual growth rate exceeding 58%, highlighting its therapeutic potential. However, limitations including hepatotoxicity, poor solubility, and slow preparation hinder its broader use, underscoring the need for safer and more effective next-generation inhibitors.
Using an integrated approach combining biophysics, structural biology, and computational biology, the authors revealed that dantrolene (DAN) and its water-soluble analogue azumolene (AZU) inhibit RyR by inducing conformational changes in the R12 domain. Surprisingly, DAN/AZU do not bind R12 alone; instead, they cooperatively bind together with intracellular nucleotides ATP/ADP within a pseudo-symmetric pocket. Direct molecular interactions between DAN/AZU and ATP/ADP drive a “clam-shell” closure of R12. This binding mode explains the ATP/ADP-dependent inhibition and suggests that cellular metabolic state may modulate drug efficacy. Together with cryo-EM data, the study further clarifies the allosteric gating mechanism by which DAN/AZU regulate RyR activity.
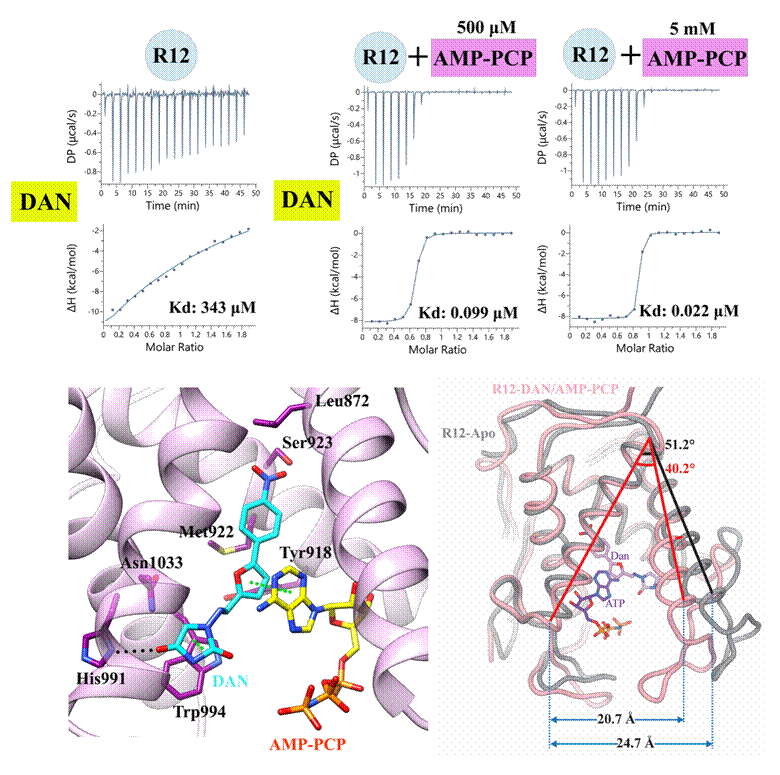
Subtype selectivity is critical for RyR-targeted drug development. This study uncovers the molecular basis of dantrolene’s subtype preference: DAN/AZU bind skeletal muscle isoform RyR1 with high affinity but much less tightly to cardiac isoform RyR2. Importantly, the key determinants of selectivity are not located within the binding pocket itself but in non-conserved residues behind the pocket that indirectly shape ligand binding, providing new insight into why drug efficacy differs across RyR subtypes.
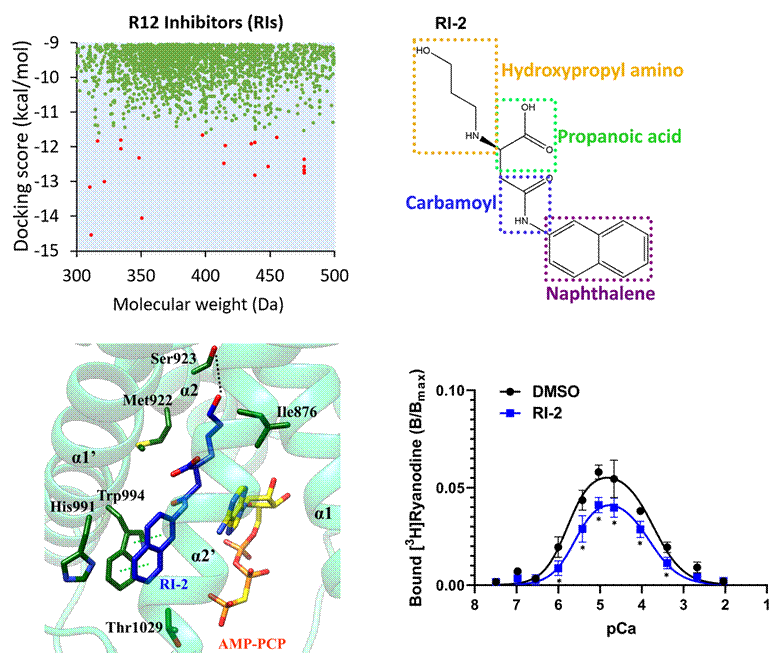
Guided by the structural information, the team performed structure-based high-throughput screening and identified a new inhibitor, RI-2. RI-2 binds the same pocket as DAN/AZU but adopts a distinct binding mode, efficiently inhibiting RyR1 activity. The crystal structure of the RI-2 complex reveals a unique interaction interface, offering a new molecular template for treating RyR channelopathies and developing RyR-targeted green insecticides.
Prof. Zhiguang Yuchi is the corresponding author of the study. PhD candidate Hadiatullah and postdoctoral researchers Lianyun Lin and Zhiyan Wang are co–first authors. This work was conducted in collaboration with the University of British Columbia (Canada), Juntendo University (Japan), Jikei University School of Medicine (Japan), and Tianjin Medical University Cancer Institute and Hospital. The study was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Haihe Laboratory of Sustainable Chemical Transformations, and received technical support from the BL18U1 and BL10U2 beamlines of the Shanghai Synchrotron Radiation Facility.

